Over the past couple of years, we have worked hard on a (new to us) material family: transition metal dihydrides. These material are crucial for applications in hydrogen-related technologies, such as energy storage, hydrogen compression, and hydrogen sensing.
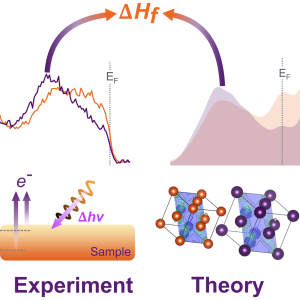
In a recently published work led by Curran, we developed a new analytical pathway to explore the relationship between chemical bonding, electronic structure and formation enthalpy of two prototypical metal dihydrides (yttrium and titanium dihydride).
Using hard X-ray photoelectron spectroscopy (HAXPES) at beamline P22 at PETRA III/DESY and by taking advantage of the tunability of synchrotron radiation, we created a non-destructive depth profile of the chemical states. We could provide a description of the bonding nature and the role of d versus sp contributions to states near the Fermi through combination of experimental valence-band spectra and insights from density functional theory (DFT) calculations, the latter was led by Dr Laura Ratcliff from the University of Bristol. Excitingly, we could determine the enthalpy of formation from both theoretical and experimental values of the energy position of metal s-band features close to the Fermi energy.
We were extra excited to see our work being highlighted by the National Research Council of Italy in a recent press release.